-
-
Humanized model of the immune system
-
iHuPBMC-T
-
iHuPBMC-NK
-
iHuPBMC-B
-
PBMC-LT
-
CD34+ HSC
-
Winn model
-
iHuPBMC-MHC/KO
-
iHuPBMC-OncVax
-
PBMC mixed inoculation model
more -
-
In vivo tumor experimental platform
-
CDX
-
iHuPDX
-
Non-GLP Toxicology
-
PK/PD
-
Brain in situ model
-
Other in situ models
-
Hematologic tumor model system inoculation
-
Creation of high interstitial tumor models
more -
-
In vitro killing experiment platform
-
Immune co-culture killing model
-
CDC
-
In vitro killing experiment platform
-
IC50
-
PDC High-Throughput In Vitro Pharmacodynamics
-
3D organoids
-
ADCC
-
T cell-mediated killing experiment
more -
-
Mouse-derived immune system model
more -
Tumor vaccine
more -
Cell therapy
more -
In vitro testing platform
more -
Non-GLP Toxicology Platform
more -
Non-tumor model and drug efficacy evaluation platform
more
-
-
-
PDX model
-
PDX model
-
Head and neck cancer
-
Eye cancer
-
Lung cancer
-
Human breast cancer
-
Esophageal cancer in humans
-
Human gastric cancer
-
Colorectal cancer
-
Human liver cancer
-
Bile duct cancer
-
Gallbladder cancer
-
Human pancreatic cancer
-
Human kidney cancer
-
Human Bladder Cancer
-
Ureteral cancer
-
Prostate cancer
-
Uterine cancer
-
Cervical cancer in women
-
Human Ovarian Cancer
-
Human skin cancer
-
sarcoma
-
Human Nervous System Cancer
-
Embryonal carcinoma
-
Human Lymphoma
-
Human leukemia
-
Multiple Myeloma
-
Adrenal gland
-
Mesothelioma
-
Other people
more -
-
CDX model
-
CDX model
-
Head and neck cancer
-
Eye cancer
-
Lung cancer
-
Human breast cancer
-
Esophageal cancer in humans
-
Human gastric cancer
-
Colorectal cancer
-
Human liver cancer
-
Bile duct cancer
-
Gallbladder cancer
-
Human pancreatic cancer
-
Human kidney cancer
-
Human Bladder Cancer
-
Ureteral cancer
-
Prostate cancer
-
Uterine cancer
-
Cervical cancer in women
-
Human Ovarian Cancer
-
Human skin cancer
-
sarcoma
-
Human Nervous System Cancer
-
Embryonal carcinoma
-
Human Lymphoma
-
Human leukemia
-
Multiple Myeloma
-
Adrenal gland
-
Mesothelioma
-
Other people
more -
-
Homogeneous Model
-
Homogeneous Model
-
Head and neck cancer
-
Eye cancer
-
Lung cancer
-
Breast cancer
-
Stomach cancer
-
Liver cancer
-
Bile duct cancer
-
Gallbladder cancer
-
Pancreatic cancer
-
Kidney cancer
-
Bladder cancer
-
Ureteral cancer
-
Prostate cancer
-
Uterine cancer
-
Cervical cancer
-
Ovarian cancer
-
Esophageal cancer
-
Skin cancer
-
sarcoma
-
Nervous System Cancer
-
Embryonal carcinoma
-
Lymphoma
-
Leukemia
-
Multiple Myeloma
-
Adrenal gland
-
Mesothelioma
-
Other
-
Colorectal cancer
more -
-
New Drug Development Services
Immunogenicity Testing
Immunogenicity Testing:
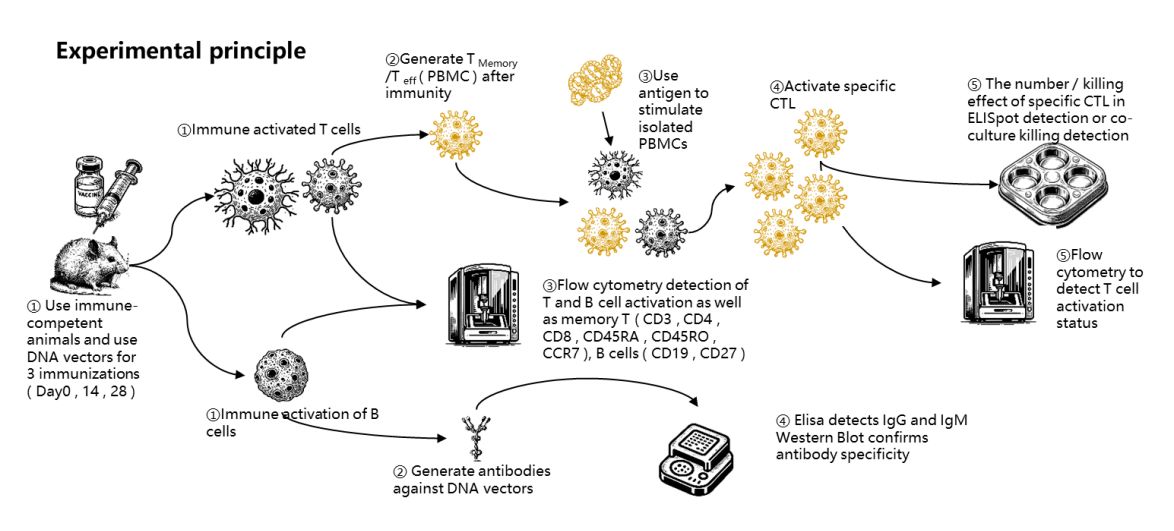
Gene therapy, tumor vaccines, and other emerging treatment options require immunogenicity testing of the vectors. Immunogenicity testing needs to assess both T cell responses and B cell responses.Model CreationUsing immunocompetent mice for immunization, T cell activation is detected through flow cytometry and Elispot methods. B cell antibody production is then detected through flow cytometry or ELISA methods, thereby confirming the immunogenicity of the test product.
Testing Content:
●T cell response: Experiments are conducted using an immunocompetent mouse model, employing flow cytometry and Elispot methods to evaluate T cell activation. Assess whether the test product can stimulate a specific T cell response.
●B cell response: Specific antibodies produced by B cells are detected through flow cytometry or ELISA methods. Detect the generation of specific antibodies induced by the test product.
●Cytokine secretion: Assess the secretion of cytokines (such as IFN-γ, IL-4, etc.) in response to stimulation by the test substance to evaluate the Th1/Th2 immune response type.
●Formation of memory immune cells: Assess whether the test product can induce long-term immune memory. T cell immune response.
Immunogenicity testing is applicable to:
Gene therapy drugs
Vaccines
Vectors
Biological drugs
Chuangmo Biotechnology (Beijing) Co., Ltd.
-
Telephone:+8615010000264 +8613810723384
-
E-mail:cndw@imodels.tech
-
Address: Building 14, Life Valley, Shuangying West Road, Changping District, Beijing
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
These cookies are necessary for basic functions such as payment. Standard cookies cannot be turned off and do not store any of your information.
These cookies collect information, such as how many people are using our site or which pages are popular, to help us improve the customer experience. Turning these cookies off will mean we can't collect information to improve your experience.
These cookies enable the website to provide enhanced functionality and personalization. They may be set by us or by third-party providers whose services we have added to our pages. If you do not allow these cookies, some or all of these services may not function properly.
These cookies help us understand what you are interested in so that we can show you relevant advertising on other websites. Turning these cookies off will mean we are unable to show you any personalized advertising.
Chuangmo Biotechnology (Beijing) Co., Ltd

