New Drug Development Services
Inflammatory bowel disease (IBD) model
Inflammatory bowel disease (IBD) is a group of chronic, relapsing inflammatory diseases of the intestines, primarily including ulcerative colitis (UC) and Crohn's disease (CD). To meet the clinical needs for research on the etiology, pathogenesis, and efficacy of newly developed drugs for this disease, various animal models have been widely used, such as chemically induced models, spontaneous models, genetically modified models, and adoptive T-cell transfer models. Among them, the dextran sulfate sodium salt (DSS)-induced animal model is the most widely used chemically induced IBD model. DSS can damage the tight junctions and basement membrane of colonic epithelial cells, thereby increasing intestinal permeability, leading to intestinal flora and other antigens entering the submucosa and systemic circulation, activating immune inflammatory responses, and inducing colitis symptoms. Animals exhibit clinical symptoms such as abdominal pain, diarrhea, hematochezia, weight loss, and fatigue. The advantages are simple operation, high success rate, good reproducibility, and its symptoms and histological changes are similar to those of human ulcerative colitis. Acute and chronic colitis models can be created by allowing animals to freely drink different concentrations of DSS aqueous solutions, making it an ideal model for studying the pathogenesis and drug efficacy of UC.
Chuangmo Biology has established a stable DSS-induced IBD disease model in C57BL/6J mice, which can be used for preclinical research and efficacy evaluation of ulcerative colitis.
- Case: DSS-induced IBD mouse disease model used to evaluate the efficacy of Ciclosporin A (CsA)
I. Prophylactic administration
Modeling and efficacy evaluation process:
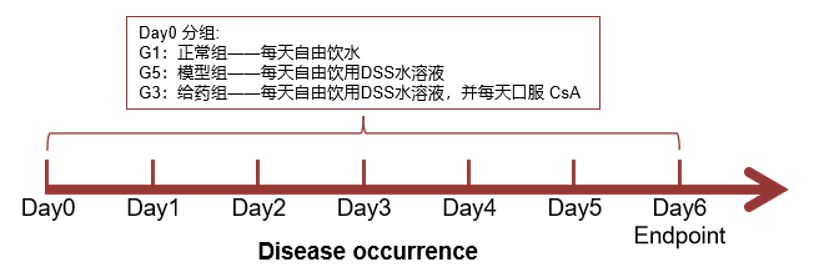
Evaluation indicators:
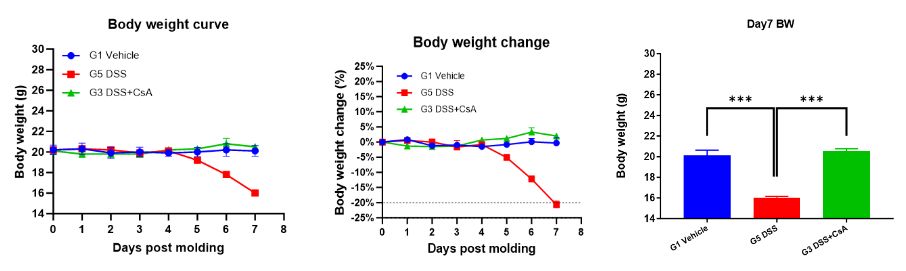
1. Body weight
Record weight changes once a day.
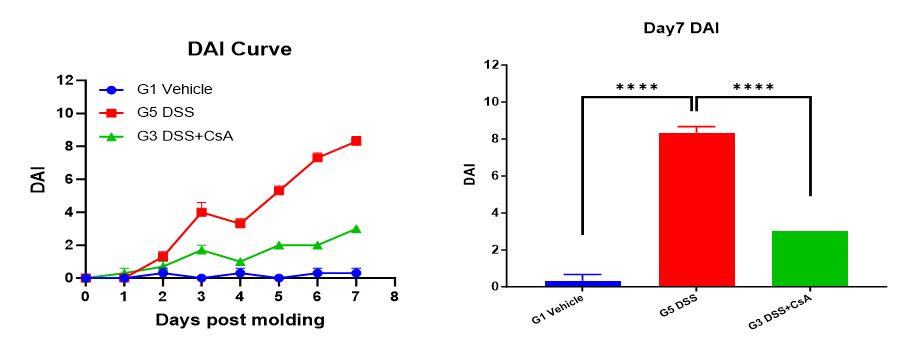
2. Disease Activity Index (DAI) score
DAI = (weight loss score + stool consistency score + bleeding score) / 3. 1) Weight loss score: Percentage of weight loss (no weight change is 0, 1-5 is 1 point, 5-10 is 2 points, 10-20 is 3 points, >20 is 4 points); 2) Stool consistency score: Stool viscosity (normal is 0, soft stool is 1 point, mucus stool is 2 points, watery stool is 3 points); 3) Bleeding score: Stool bleeding (normal 0 points, light blue occult blood indicator is 1 point, blue occult blood indicator is 2 points, dark blue occult blood indicator is 3 points, gross blood in stool is 4 points).
DAI can be used for quantitative analysis of the disease condition in mice. Score the mice once a day for DAI.
3. Colon length
Measure colon length at the end of the experiment.

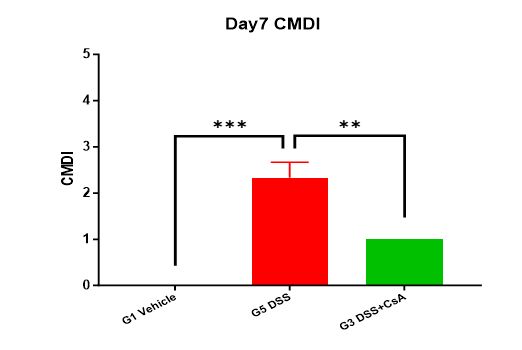
4. Visual colonic mucosal damage (CMDI) score
At the end of the experiment, score the mice for CMDI. CMDI scoring standard: No damage is 0 points; mild congestion, edema, smooth surface, no erosion or ulceration is 1 point; congestion, edema, rough granular mucosa, erosion or intestinal adhesion is 2 points; severe congestion, edema, mucosal surface necrosis and ulcer formation, maximum ulcer longitudinal diameter <1.0 cm, intestinal wall thickening or surface necrosis and inflammation is 3 points; based on 3 points, the maximum ulcer longitudinal diameter is >1.0 cm or the entire intestinal wall necrosis is 4 points.
5. Pathological examination
After the experiment, collect colon tissue for HE staining and pathological examination.
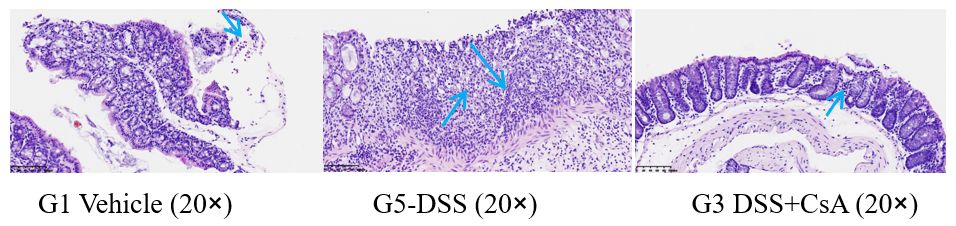
G1 Vehicle: A small number of lymphocytes are scattered in the lamina propria of the intestinal mucosa, and a small number of free neutrophils are observed.
G5-DSS: Focal ulceration of the intestinal mucosa, inflammatory exudate and granulation tissue hyperplasia are visible, and many inflammatory cells (including many eosinophils, lymphocytes, neutrophils, histiocytes, etc.) and lymphoid follicles are observed.
G3 DSS+CsA: A small number of lymphocytes are scattered in the lamina propria of the intestinal mucosa, and a small amount of free pancreatic tissue is observed.
Summary: Experimental data show that compared with the unmodeled group (G1 Vehicle), the modeled group (G2 DSS) showed a decrease in animal weight, and the DAI and CMDI scores were significantly increased. A significant shortening of colon length was detected on Day 7; ulcer formation showed inflammatory exudate and granulation tissue hyperplasia, with a large number of inflammatory cell infiltration and lymphoid follicles. The drug-treated group (G3 DSS+CsA) showed no significant change in animal weight; DAI and CMDI scores were slightly increased; colon length was shortened on Day 7, but the degree of shortening was less than that of the modeled group (G2 DSS); pathological results showed significant alleviation of colon symptoms, similar to the pathological structure of the unmodeled group (G1-Vehicle). This indicates that the DSS-induced C57BL/6 mouse inflammatory bowel disease model was successfully modeled, and the prophylactic CsA treatment regimen significantly alleviated the clinical symptoms of the DSS-induced C57BL/6 mouse inflammatory bowel disease model.
II. Therapeutic administration
Modeling and efficacy evaluation process:
Evaluation indicators:
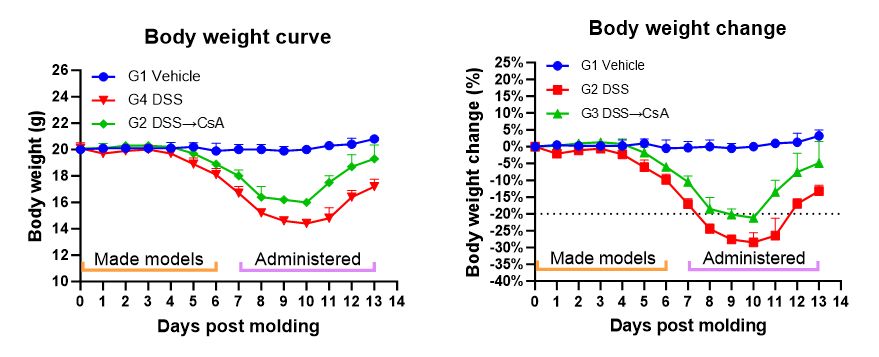
1. Body weight
Record weight changes once a day.
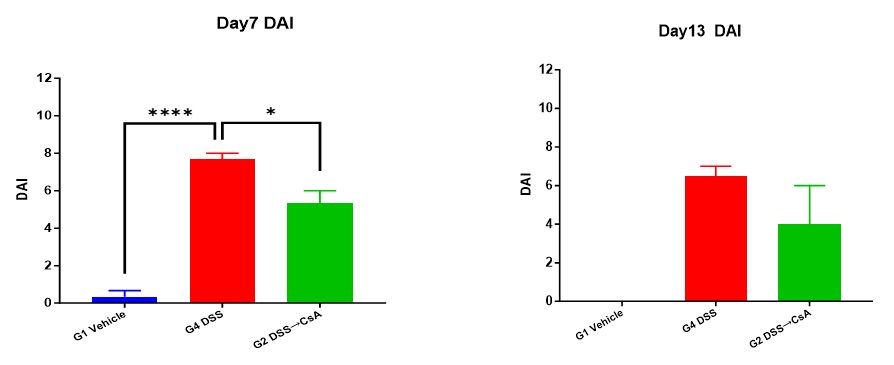
2. Disease Activity Index (DAI) score
At the end of the experiment, score the mice for DAI.
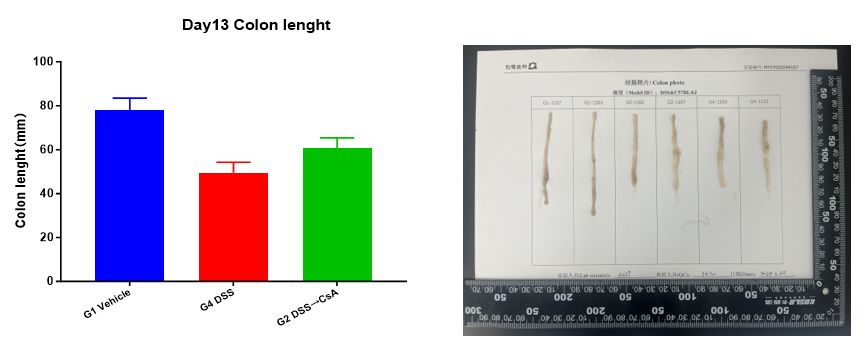
3. Colon length
Measure colon length at the end of the experiment.
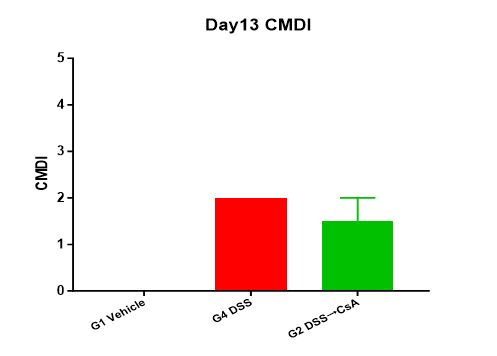
4. Visual colonic mucosal damage (CMDI) score
At the end of the experiment, score the mice for CMDI.
5. Pathological examination
After the experiment, collect colon tissue for HE staining and pathological examination.
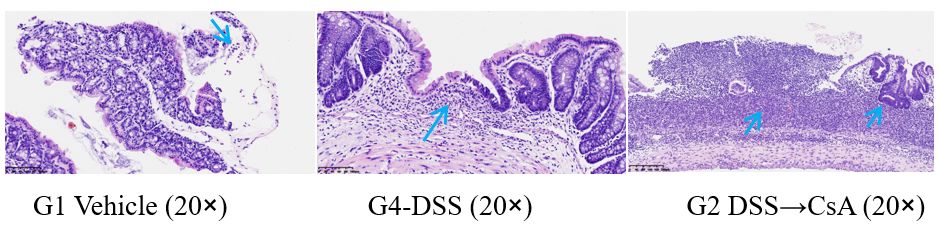
G1 Vehicle: A small number of lymphocytes are scattered in the lamina propria of the intestinal mucosa, and a small number of free neutrophils are observed.
G4 DSS: The intestinal mucosa is significantly thinned locally, and no large intestinal glands are observed in this area of the lamina propria. Slightly more inflammatory cells (lymphocytes, eosinophils, etc.) are observed, and focal mucosal defects are also observed. No significant inflammatory reaction or granulation tissue is observed in this area (considered an artifact), and lymphoid follicles are also observed.
G2 DSS→CsA: Local ulceration of the intestinal mucosa, inflammatory exudate and granulation tissue hyperplasia are visible, and a large number of inflammatory cells (including a large number of neutrophils, lymphocytes, histiocytes, etc.) and lymphoid follicles are observed. Some glandular epithelium shows hyperplastic changes, and some glandular epithelium shows low-grade intraepithelial neoplasia.
Summary: Experimental data shows that in the modeling group (G4 DSS), compared to the unmodeled group (G1 Vehicle), the animals' weight continuously decreased during the modeling process. After stopping DSS stimulation on Day 7, the weight recovered; the DAI score on Day 13 was also significantly lower than that on Day 7; compared to the modeling group (G5 DSS) on Day 7 in the prophylactic administration experiment, the colon length increased on Day 13, and the CMDI score slightly decreased (individual differences cannot be ruled out); pathological results showed massive inflammatory cell infiltration, thinning of the intestinal mucosa, and ulcer formation. In the administration group (G2 DSS→CsA), the animals' weight continuously decreased during the modeling process. After stopping DSS stimulation on Day 7 and continuous administration, the weight recovered, and the recovery degree was greater than that in the modeling group (G4 DSS); the DAI score on Day 13 was also significantly lower than that on Day 7; compared to the administration group (G3 DSS+CsA) on Day 7 in the prophylactic administration experiment, the colon length increased on Day 13, and the CMDI score was slightly higher; pathological results showed similarities to the modeling group, but also showed partial hyperplasia and repair changes in the glandular epithelium. This indicates that the therapeutic CsA administration regimen alleviates weight loss in the DSS-induced inflammatory bowel disease model in C57BL/6 mice. Other clinical symptoms, such as stool viscosity, hematochezia, colon length, and the degree of intestinal mucosal damage, did not show significant relief in the administration group compared to the modeling group. The relief of symptoms in both groups from Day 7 to 13 mainly depends on stopping DSS stimulation.
In summary, CsA has a certain therapeutic effect in the DSS-induced IBD mouse disease model, but prophylactic administration is more effective.

